Electrophysiology
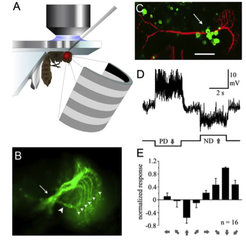
Nerve cells transmit information by means of rapidly changing electrical signals. Those can be recorded with sub-millisecond precision using electrophysiological techniques such as whole-cell patch-clamp recordings. We perform electrophysiological recordings from single neurons in the fly visual system to measure their responses to visual stimuli. In addition, using Drosophila as a model system, we can combine such measurements with precise circuit manipulations using the powerful genetic toolbox available in this organism. We can for instance silence selected elements and assess the effect of this manipulation on the neural responses of downstream cells. To this end, synaptic silencers, toxins, potassium channels or light-gated chloride channels can be expressed (shibirets, tetanus toxin, Kir2.1, GtACR1). We can also activate specific neurons using light-gated cation channels (channelrhodopsin) and thus precisely probe synaptic connectivity. From the effects of such manipulations, the information flow in neural cicuits can be resolved in great detail.
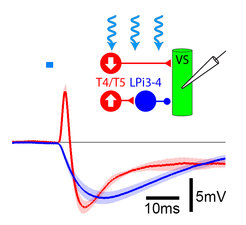
Such approaches revealed for instance that lamina neurons L1 and L2 represent the major input channels to the motion detection circuit. Whereas L1 transmits information about brightness increments (ON pathway) only, L2 signals information about brightness decrements (OFF pathway) to downstream neurons (Joesch et al., 2010). Subsequently, these findings led to the discovery that visual motion is detected by two parallel motion detectors, one within each pathway (Eichner et al., 2011; Maisak et al., 2013, Joesch et al., 2013). The output elements of the ON and OFF pathways are constituted by the T4 and T5 cells, respectively (Maisak et al., 2013). T4 and T5 cells with the same tuning provide excitatory synaptic input to wide-field tangential cells, which thus obtain visual signals related to ego-motion (Joesch et al., 2008; Schnell et al., 2010; Schnell et al., 2012; Mauss et al., 2014). Shown by electrophysiology, genetic silencing and optogenetic stimulation, tangential cells also receive indirect inhibitory input from oppositely tuned T4/T5 neurons via LPi cells, giving rise to null direction inhibition and enhancing response selectivity (Mauss et al., 2015).
Tangential cells in the lobula plate do not work independently but possess multiple chemical and electrical connections with each other to form an intricate neural network (Borst & Weber, 2011). These interactions have been studied by performing dual electrophysiological recordings, dye-coupling and laser ablation experiments in blow flies. A major outcome of these studies is that the receptive fields of tangential cells are not only determined by input from retinotopic motion detectors, but are broadened and shaped by lateral interactions among themelves.
References
Borst, A. & Weber, F. Neural action fields for optic flow based navigation: a simulation study of the fly lobula plate network. PLoS ONE 6, e16303 (2011).
Eichner, H., Joesch, M., Schnell, B., Reiff, D. F. & Borst, A. Internal structure of the fly elementary motion detector. Neuron 70, 1155–1164 (2011).
Joesch, M., Weber, F., Eichner, H. & Borst, A. Functional specialization of parallel motion detection circuits in the fly. J. Neurosci. 33, 902–905 (2013).
Joesch, M., Schnell, B., Raghu, S. V., Reiff, D. F. & Borst, A. ON and OFF pathways in Drosophila motion vision. Nature 468 , 300–304 (2010).
Joesch, M., Plett, J., Borst, A. & Reiff, D. F. Response properties of motion-sensitive visual interneurons in the lobula plate of Drosophila melanogaster. Curr. Biol. 18, 368–374 (2008).
Maisak, M. S. et al. A directional tuning map of Drosophila elementary motion detectors. Nature 500, 212–216 (2013).
Mauss AS, Meier M, Serbe E, Borst A (2014) Optogenetic and pharmacologic dissection of feedforward inhibition in Drosophila motion vision. J Neurosci 34: 2254-2263.
Mauss AS, Pankova K, Arenz A, Nern A, Rubin GM, Borst A (2015) Neural circuit to integrate opposing motions in the visual field. Cell 162: 351-362.
Schnell, B., Raghu, S. V., Nern, A. & Borst, A. Columnar cells necessary for motion responses of wide-field visual interneurons in Drosophila. J. Comp. Physiol. A 198, 389–395 (2012).
Schnell, B. et al. Processing of horizontal optic flow in three visual interneurons of the Drosophila brain. J. Neurophysiol. 103, 1646–1657 (2010).

